-
Common NameThiophanate-methyl
-
中文通用名甲基硫菌灵
-
IUPACdimethyl 4,4′-(o-phenylene)bis(3-thioallophanate)
-
CASdimethyl [1,2-phenylenebis(iminocarbonothioyl)]bis[carbamate]
-
CAS No.23564-05-8
-
Molecular FormulaC12H14N4O4S2
-
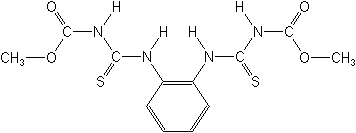
Molecular Structure
-
Category
-
ActivityFungicide, wound protectant
-
PremixTricyclazole+Thiophanate-methyl
Thiophanate-methyl+Zinc thiazole
Thiophanate-methyl+thiram
Thiophanate-methyl+Tebuconazole
Thiophanate-methyl+sulfur
Thiophanate-methyl+Penconazole
Thiophanate-methyl+Fluazinam
Thiophanate-methyl+Ethirimol
Thiophanate-methyl+diethofencarb
Pyraclostrobin+thiophanate-methyl
Imidacloprid+mancozeb+thiophanate-methyl
Dithianon+Thiophanate-methyl
Cyprodinil+Thiophanate-methyl
Chlorothalonil+thiophanate-methyl
Azoxystrobin+metalaxyl+thiophanate-methyl
Wettable powder, paste, flowable, granule, dust, ULV. Premix Parters: thiram;
-
Physical PropertiesMolecular weight:342.4; Physical form:Colourless crystals. Melting point:172 °C (decomp.); Vapour pressure:0.0095 mPa (25 °C); Partition coefficient(n-octanol and water):logP = 1.50; pKa:7.28; Solubility:Practically insoluble in water (23 °C). In acetone 58.1, cyclohexanone 43, methanol 29.2, chloroform 26.2, acetonitrile 24.4, ethyl acetate 11.9 (all in g/kg, 23 °C). Slightly soluble in hexane.; Stability:Stable in neutral, aqueous solution at room temperature. Stable to air and sunlight. Quite stable in acidic solution at room temperature; unstable in alkaline solution; DT50 24.5 h ( pH 9, 22 °C). Formulated prod;
-
ToxicologyOral:Acute oral LD50 for male rats 7500, female rats 6640, male mice 3510, male rabbits 2270 mg/kg. Percutaneous:Acute percutaneous LD50 for male and female rats >10 000 mg/kg. Mild skin and eye irritant. Inhalation: LC50 (4 h) for rats 1.7 mg/l air. ADI:( JMPR) 0.003 mg/kg b.w. [1979].
-
Environmental ProfileEcotoxicology:
Algae:EC50 (96 h) for Chlorella 0.8 mg/l.Bees:Not toxic to bees; LD50 (topical) >100 µg/bee.Birds:Acute oral and percutaneous LD50 for Japanese quail >5000 mg/kg.Daphnia:LC50 (48 h) 20.2 mg/l.Fish:LC50 (48 h) for rainbow trout 7.8, carp 11 mg/l.
Environmental fate:
Animals:In rats, following oral administration, 61% is excreted in the urine and 35% in the faeces within 90 minutes after the last dose. Metabolism involves cyclisation to carbendazim ( q.v.). The principal metabolite in rats is methyl 5Soil:Soil persistence is c. 3-4 weeks. In soil, in aqueous solution, and under the influence of u.v. light, cyclisation occurs, leading to the formation of carbendazim (q.v.). This then undergoes degradation Plant:In plants, cyclisation occurs, leading to the formation of carbendazim ( q.v.). -
Transport InformationSignal Word:CAUTION; Hazard Class:III(Slightly hazardous)
Porduct NewsMore
Canada proposes to re-evaluate thiophanate-methyl
IHARA launches seed treatment product CERTEZA N for control of nematode in Brazil
EU proposes not to renew approval of fungicide thiophanate-methyl
IHARA launches fungicide Approve in Brazil
Cheminova fungicide Cercobin receives registration in California
China permits manufacturing of thiophanate-methyl and more ais
Related CompaniesMore
Country: India
Wokozim™ Granule Wokozim™ Liquid Wokozim™ Drip Wokozim™ Seed Plus Wokozim™ Home & Garden Sulphur Thiophanate-methyl Acetamiprid Hexythiazox Carbendazim + Mancozeb
Shanghai Nicex International Trading Co.,Ltd.
Country: China
Oxadiazon Oxadiargyl Azoxystrobin TC Bentazone 2,4-D TC MCPA TC Nicosulfuron Tribenuron-methyl Bispyribac-sodium
Ningxia Lamtin Agricultural Development Co.Ltd.
Country: China
Oxadiargyl Bentazone Oxadiazon Metamifop Propanil
Shandong Zhongxin Kenong Biological Technology Co., Ltd
Country: China
Lambda-cyhalothrin Imidacloprid Acetamiprid Emamectin benzoate Abamectin Pymetrozine Propiconazole Iprodione Carbendazim+mancozeb Azoxystrobin+difenoconazole Diquat
Anhui Huaxing Chemical Industry Co., Ltd.
Country: China
Kresoxim-methyl Cartap Glyphosate Imidacloprid Nicosulfuron MCPA-2-ethylhexyl Flusilazole Thiosultap-monosodium MCPA Fipronil

 0
0 Subscribe
Subscribe